
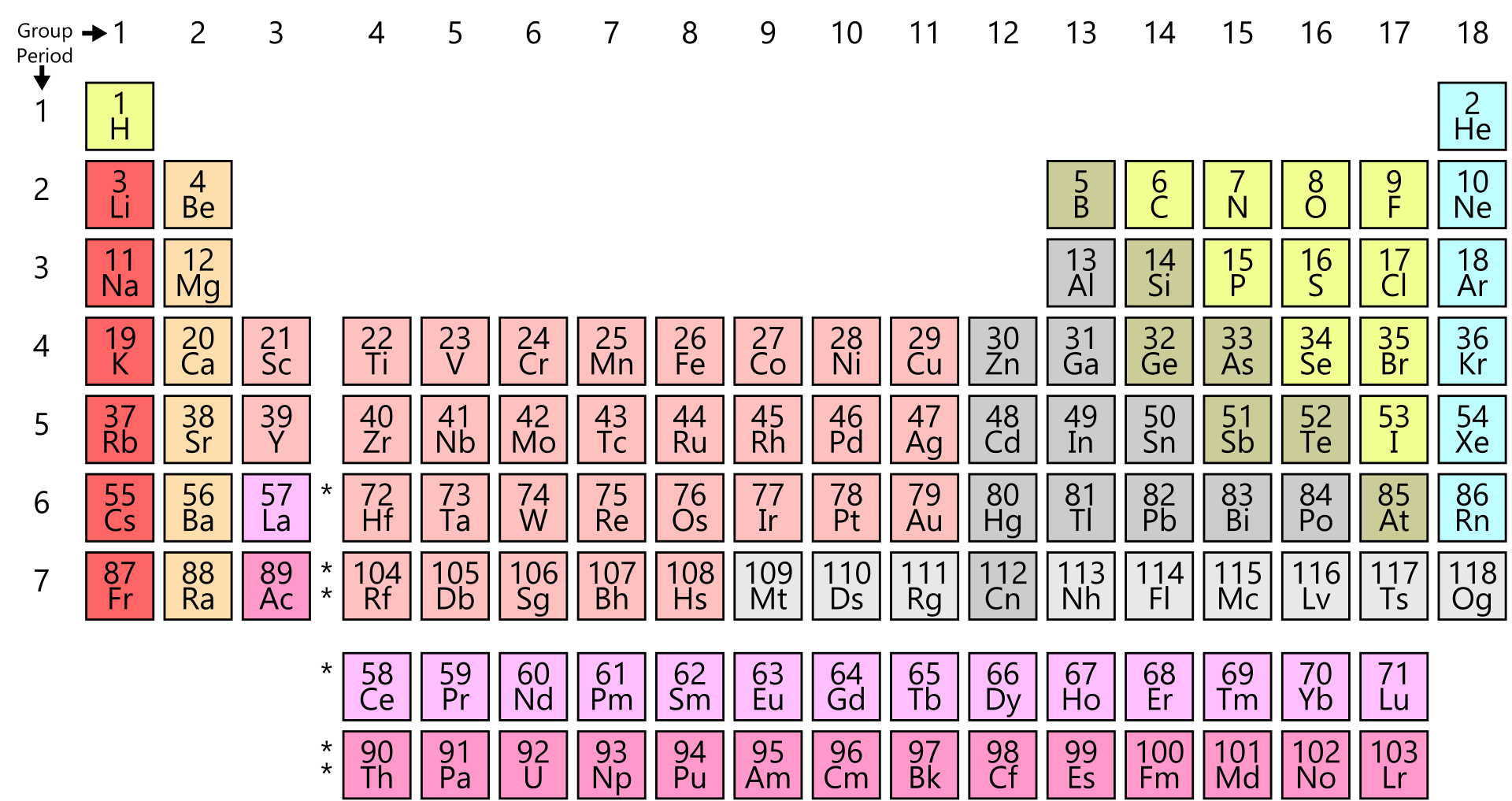
Note: There are Bohr Diagram cards for elements 1 (hydrogen) through 20 (calcium) included in the Chemistry Packet. each column/group had the same number of electrons in the outer ring (except for helium).each row/period added on “shell” (or ring) around the nucleus.Then, the kids had to observe the kids and see if they could find any trends: I divided the cards among the kids and had them place the cards on top of the cards below.

I handed the kids Bohr Diagram cards that were the same size as the element cards from the activity above. We did another hands-on activity to introduce the kids to valence electrons. Next we went over the parts of the atom (nucleus, protons, electrons, nucleus, etc.). Note: There are cards for the elements 1 (hydrogen) through 36 (krypton) included in the Chemistry Packet. They also saw that the yellow cards all had one little electron in the lower left corner. (For example, they pretty quickly figured out that the blue elements were all metals - like iron, copper, zinc, etc.). The teacher notes (pictured below right) explain some of the hints I gave them on the way, but they did a great job with it as you can see from the pictures above! Along the way, the students took notes on the observation page (seen below in the middle) about the families they were looking at. I told them that Mendeleev knew quite a bit about the properties of the various elements… and with their color-coded cards, they had considerable information too. We talked about Mendeleev, who was the first to develop the periodic table and discussed how challenging that must have been. My daughter spent several days coloring the cards (while we did read alouds). This activity includes the first 36 elements.
The periodic table chemistry unit how to#
In the packet, there is a student instruction sheet on how to color the elements. This was the first activity we did as we started looking closely at the Periodic Table: We have the Molecular Model Kit (affiliate link) for building molecules.My 5th grader also spent some time building molecules. Plus, there was some space for her to write some basic facts about each element. This booklet had her find out: the atomic number, atomic weight, period and group numbers. It has huge pictures of the elements as well as pictures of items that use this element. She used a beautiful book about the elements by Theodore Gray Elements: A Visual Exploration of Every Known Atom in the Universe ( affiliate link) as a reference. My youngest was in 5th grade she worked on these pages. Update: There are two new chemistry booklets on the first 20 elements and on elements with unusual symbols in this packet.
The periodic table chemistry unit free#
(She still wanted to participate but acknowledged it was challenging for her!) Be sure to this post Fun, Simple Science Experiments for Younger Kids! (Introducing Kids to Chemistry) if you have younger kids (It has a free Chemistry Experiment Packet for young learners.) The First 20 Elements and Elements with Unusual Symbols:

Note: Chemistry for Elementary - My youngest enjoyed the unit, but some of the topics we touched on as the unit went on were a bit above her head. I think this unit is best for middle school and up (perhaps as a supplement to for high schoolers being introduced to chemistry for the first time). We did this unit together when the kids were 10, 12 and 14. Atomic Number, Atomic Mass & Chemical Symbols.hydrogen & the alkali metals, alkaline Earth metals, halogens, noble gases.Bohr Diagrams & Understanding Valence Electrons.There you can find the metals, semi-conductor(s), non-metal(s), inert noble gas(ses), Halogens, Lanthanoides, Actinoids (rare earth elements) and transition metals.I am excited to share our new chemistry unit with you! (It has been recently updated and now is around 150 pages!!) As you know, we love hands-on activities and I want to show you some of the fun ways we explored the periodic table and touched on topics like valence electrons, Bohr Diagrams, Lewis Diagrams (electron dot diagrams), ions, isotopes, and more! Please note that the elements do not show their natural relation towards each other as in the Periodic system. The unity for atomic mass is gram per mol. The lightest chemical element is Hydrogen and the heaviest is Hassium. The chemical elements ofįor chemistry students and teachers: The tabular chart on the right is arranged by Atomic mass (weight). This list contains the 118 elements of chemistry. Separation and Concentration Purification RequestĬhemical elements listed by atomic mass The elements of the periodic table sorted by atomic massĬlick on any element's name for further information on chemical properties, environmental data or health effects.Plant Inspection & Process Optimalisation.


 0 kommentar(er)
0 kommentar(er)
